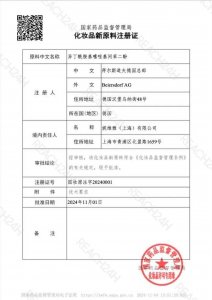
REACH24H Secures Registration of Thiamidol, First Whitening Ingredient Under China’s CSAR
HANGZHOU, November 7, 2024 – On November 4, 2024, the National Medical Products Administration (NMPA) of China officially approved Isobutylamido Thiazolyl Resorcinol (Thiamidol 630), an innovative whitening ingredient developed by Beiersdorf Group. This marks the first whitening ingredient to be approved under the Cosmetic Supervision and Administration Regulation (CSAR), a key milestone for the industry.
REACH24H Consulting Group is proud to have supported Beiersdorf Group with expert guidance on cosmetics regulatory compliance and technical assistance, contributing to this achievement.
About Isobutylamido Thiazolyl Resorcinol (Thiamidol 630)
Isobutylamido Thiazolyl Resorcinol (Thiamidol 630) is a whitening cosmetic ingredient developed by Beiersdorf Group. Synthesized through a chemical process, Isobutylamido Thiazolyl Resorcinol has undergone thorough efficacy and safety assessments. Approved for use in leave-on cosmetics (excluding products that may pose inhalation risks), it opens new opportunities for the development of freckle removal and skin-whitening products in China. This milestone is expected to inspire further research and drive ingredient innovation across the industry.
Whitening Ingredients Compliance in China
According to the Provisions for Management of New Cosmetic Ingredient Registration and Notification Dossiers, freckle-removing and whitening ingredients can be registered under two situations:
- Situation 1: For new cosmetic ingredients intended for functions of preservative, sunscreen, coloring, hair dyeing, freckle-removing and whitening, anti-hair loss, anti-acne, anti-wrinkle (except for physical anti-wrinkle), anti-dandruff, deodorant efficacy and other new cosmetic ingredients with higher biological activity that are used for the first time at home and abroad, the above-mentioned toxicological test documents from Items 1 to 12 shall be submitted.
- Situation 4: For new cosmetic ingredients that are intended for functions of preservative, sunscreen, coloring, hair dyeing, freckle-removing and whitening, anti-hair loss, anti-acne, anti-wrinkle (except for physical anti-wrinkle), anti-dandruff, deodorant efficacy and provided with sufficient evidence proving that such ingredient has safety use history of more than three years in cosmetics marketed overseas, the above-mentioned toxicological test documents from Items 1 to 7 shall be submitted.
REACH24H’s Expertise in Cosmetic Ingredients Compliance
REACH24H Consulting Group is honored to have guided Beiersdorf Group through the regulatory process for Thiamidol 630 in China. As a leading compliance service provider, REACH24H has supported over 1,000 companies worldwide in navigating complex cosmetics regulations. With over 20 successful new ingredient registrations/notifications, our team continues to deliver exceptional regulatory consulting services that ensure compliance with both local and international standards, helping companies successfully launch their products in global markets.
For inquiries or more information about cosmetic products and ingredients compliance, please contact customer@reach24h.com.