Korea REACH

What is K-REACH?
Korea REACH (K-REACH) refers to ‘The Act on Registration and Evaluation, etc of Chemical Substances’, which was promulgated by the Ministry of Environment of Korea (MoE) and came into force on 1 Jan 2015. The Act is well-known as Korea REACH due to its similarity to the EU REACH regulation. Early in the year of 2016, Korea MoE initiated the amendment of the Act, and on 28 Feb 2018, the newly revised K-REACH regulation was approved by National Assembly and finally implemented on 1 Jan 2019. Under current K-REACH, any company who intends to manufacture or import a new chemical substance for greater than 0.1 t/a or existing chemical substance for greater than 1 t/a shall do registration before manufacture or import. Additionally, K-REACH also adopts the concepts of pre-registration under EU REACH, which requires all >=1t/a existing chemical substances to apply for pre-registration between 1 Jan 2019 to 30 June 2019 to benefit from the grace periods.
How to comply with K-REACH?
K-REACH uses the Only Representative (OR) concept and allows non-Korean companies to register through an OR on their behalf. The Ministry of Environment (MoE) is responsible for the registration and evaluation of chemical substance under K-REACH.
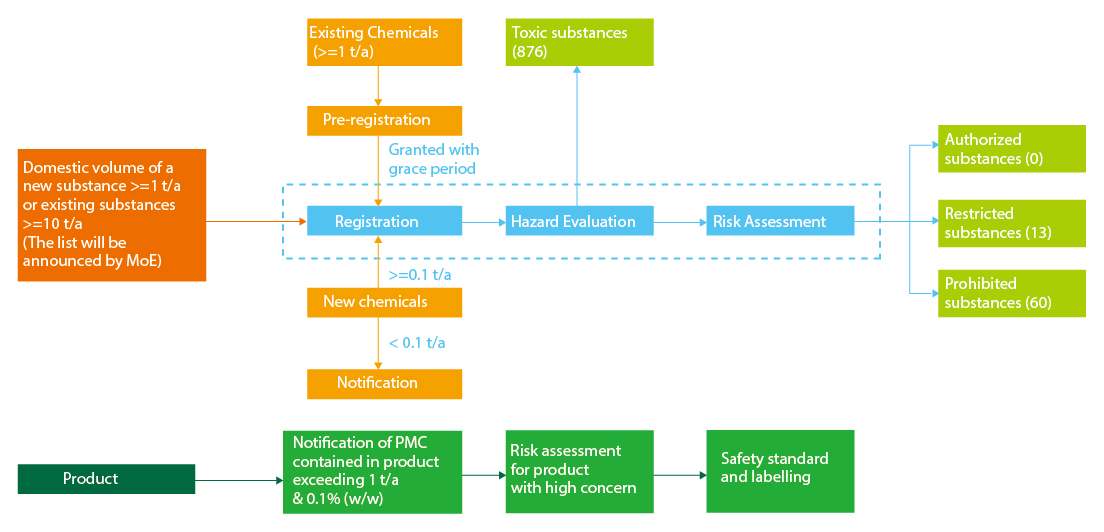
Registration
Substances Subject to Registration
1. New chemical substances (>=0.1 t/a)
2. Existing chemical substances (>=1 t/a)
3. New chemical substances and existing chemical substances designated by MOE for their total domestic volume exceeds the standard set by the Presidential Decree (total 1 t/a for new chemicals, total 10 t/a for existing chemicals)
Registration Types
Existing chemicals: Pre-registration, Joint Registration
1. Pre-registration
Companies who manufacture or import existing chemical substances for greater than 1 t/a shall do pre-registration between 1 Jan 2019 to 30 Jun 2019 to benefit from the grace periods.
2. Joint Registration
After pre-registration, companies will be granted with a certain grace period for their chemicals according to the hazard and tonnage band. They need to do joint registration on same chemicals before the grace period expires, which is similar to EU REACH.
New chemicals: Simplified Registration, Standard Registration
1. Simplified Registration
0.1-1t/a new substances before 31 Dec 2019.
2. Standard Registration
>= 1 t/a new substances & 0.1-1t/a new substances after 31 Dec 2019
Grace Period
1. New substances don’t have grace period, all new chemical substances should be subject to registration prior to manufacture or import.
2. The grace period for 510 PECs has expired since 1 July 2018, now registration of 510 PECs should be finished before manufacture or import.
3. The grace period for existing substances (exclude the 510 PECs) is as below.

Notification
1. New substances < 0.1 t/a
2. Substances that have received exemption approval under previous ‘Toxic Chemical Control Act’ (TCCA):
– New substances under 0.1 t/a
– New polymers which is composed of existing monomers and meet one of below criteria:
1) Polymer with Mn over 10,000 D which contains oligomers with molecular weight of less than 1,000 is over 5%, or oligomers with molecular weight of less than 500 is over 2%.
2) Polymer with Mn between 1000-10,000 D which contains oligomers with molecular weight of less than 1,000 is over 25%, or oligomers with molecular weight of less than 500 is over 10%.
3) Cationic polymer
4) Polymer with Mn under 10,000, which contains residual monomer of hazardous chemical, or priority management chemical exceeding 0.1%.
Registration Exemption Application
-Chemical substance imported/manufactured for export-only use, including substance imported/manufactured to make export-only products
-Chemical reagents
-Surface treated substances
-Non-isolated intermediates or on-site isolated intermediates which can be technically blocked from leakage or exposure
-Polymer of low concern (PLC)
-Substances for R&D use
Risk Assessment
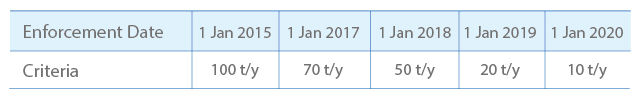
1. Risk assessment is required for substances manufactured or imported for 10 tons or more per year
2. Depending on annual tonnage, the deadlines of submitting risk assessment are listed as follows:
Product Notification
Subject to Product Notification
Priority Management Chemicals (PMC) are present in a product exceeding a specific threshold (above 1 ton/year & 0.1% w/w).
What is PMC?
Priority Management Chemicals (PMC) mean risk-posing chemical substances which fall under any of the following criteria. Now PMC list has been announced by MoE which includes two sub-lists with 672 substances in total. The first list (204 substances) will come into force on 1 July 2019. The second list (468 substances) will come into force on 1 July 2021.
-CMR substances and substances with endocrine disrupting properties (EDC)
-Bio-accumulative and persistent substances (vPvB or PBT)
-Substances which may damage organs such as lungs, kidneys after repeated exposure (STOT)
-Other substances which may give the same level of concern
What to Notify?
1. Notifier information
2. Chemical information of PMC
3. Content and hazard information of PMC
4. Exposure information
5. Uses 6. Classification and label
Penalty and fine under K-REACH
Imprisonment for not more than 5 years or by a fine not exceeding 100 million won would be applied to person who does not comply with the relevant obligations under K-REACH. The fine for violation may also imposed on the basis of total sales of the company (no more than 5%) according to the amended K-REACH.
The Ways to Comply with K-REACH
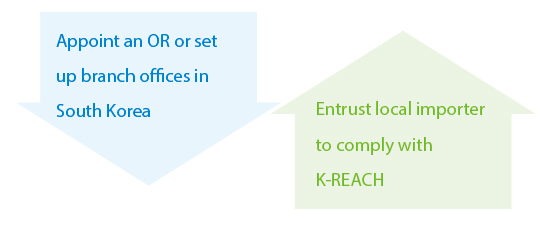
Note: Non-korean companies can prevent their valuable substance information from being disclosed to the public and their importers in Korea while appointing an OR.
Supply Chain Communication
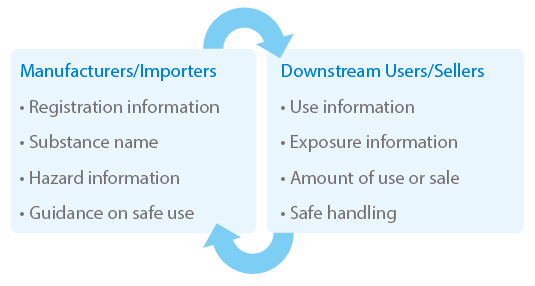
Note: The Ministry of Employment and Labor (MOEL) requires companies exporting chemicals and products into Korea to provide Korea SDS and labeling.
Our Services
- Only Representative Service
- New Chemical Substance Search
- Registration Dossiers Preparation and Submission
- Lead Registrant
- Pre-registration
- Data Sharing
- Risk Assessment Report Preparation and Submission
- Product Notification
- Exemption Application
- Training and Consultancy on K-REACH
- Korea Safety Data Sheet (SDS) and Label Preparation
