China’s Cosmetic Ingredient Submission Code
Overview
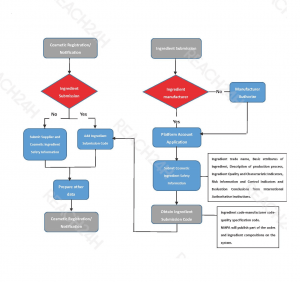
According to the Technical Guidance for Submission of Cosmetic Ingredient Safety Information, the information on the safety of Ingredient quality should include:
- Ingredient trade name
- Basic attributes of ingredient
- The suggested percentage used in cosmetics
- Description of production process
- Ingredient Quality and characteristic indicators
- Risk information and control indicators (when necessary)
- Evaluation conclusions from International Authoritative Institutions
If the suppliers fill in the above information and submit it successfully, they will obtain the exclusive ingredient submission code.
The ingredient submission code consists of three parts: ingredient code-manufacturer code-quality specification code.
Competent Authority in China
Administrative Department
National Medical Products Administration (NMPA)
Relevant Regulations
·Technical Guidance for Submission of Cosmetic Ingredient Safety Information
·Inventory of Prohibited Ingredients for Cosmetics
- Inventory of Existing Cosmetic Ingredients in China 2021
- Notice on Transitional Measures on the Existing New Cosmetic Ingredients Applications (No.284)
- Provisions for Management of New Cosmetic Ingredient Registration and Notification Dossiers
Submission Process of Ingredient
Our Services
New Cosmetic Ingredient (NCI) Registration / Notification
- Regulation Consulting Service
- Platform Account Application
- Dossier Preparation and Submission
- Follow-up with NMPA’s Comments
Toxicological Data Gap Analysis and Ingredient Application Proposal
- Ingredient Pre-Evaluation
- Data Gap Analysis
- NCI Application Budget and Leadtime Calculation
- NCI Application Strategy Formulation
Supplementary Materials Submission after New Cosmetic Ingredient Technical Review
- Submit Supplementary Materials Based on Technical Review Comments
- Follow up with the Comments from Technical Review Panels
Testing Monitoring for New Cosmetic Ingredient Application
- Laboratory Selection
- Experiment Proposal and Arrangement
- Testing Monitoring
- Test Report Review
Ingredient Safety Assessment Report
- Ingredient Primary Information Confirmation
- Toxicological Data Analysis and Evaluation
- Safety Assessment Report Writing
Toxicological Data Gap Analysis and Ingredient Application Proposal
- Ingredient Pre-Evaluation
- Data Gap Analysis
- NCI Application Budget and Leadtime Calculation
- NCI Application Strategy Formulation