The EU and US Biocide Regulation Trends in the Times of Covid-19
The covid-19 pandemic has increased the demand for biocidal products across the world. For example, in the UK, disinfectant sales have risen 255% year-to-year in February 2020. However, these products are highly regulated in most parts of the world. During a special session of the 12th Chemical Regulatory Annual Conference & Asian Helsinki Chemicals Forum on December 4, Mr. Klaus Berend from the European Commission and Dr. Shannon L. Gainey from REACH24H USA offered a comprehensive introduction to the EU and US biocide regulation.
EU BPR Framework
The EU Biocidal Products Regulation 528/2012 covers a wide range of products intended to destroy, deter, rendering harmless, prevent or otherwise exert a controlling effect on, any harmful organism by any means other than mere physical or mechanical action. Furthermore, articles with deodorizing or antimicrobial claims that have been treated with, or intentionally incorporating, one or more biocidal products need to comply with the regulation.
Before entering the EU market, a biocidal product needs to complete two steps. First, the active substances contained in that biocidal product must be previously approved. Secondly, the product must get authorization before it can be sold on the market. To obtain approval of active substance, the applicant needs to submit ECHA sufficient safety and efficacy data on the substance. If an active substance was categorized as CMR category 1A or 1B, BPT, vPvB, or endocrine disruptor, it would not be approved. The product authorization can be obtained in different ways, such as unified authorization that enable the full market access all at once, or simplified authorization of products using active substances listed in Annex I of the regulation. Enterprises may choose one of these approaches depending on the market demand and product features.
Enforcement progress
From an active substance perspective, ECHA has launched the substance review program as early as 2000, aimed to review all existing active substance by 2024, but the progress is disappointingly slow, as only 35% of the substances have been reviewed. According to Mr. Klaus Berend, most of the active substances have passed the review.
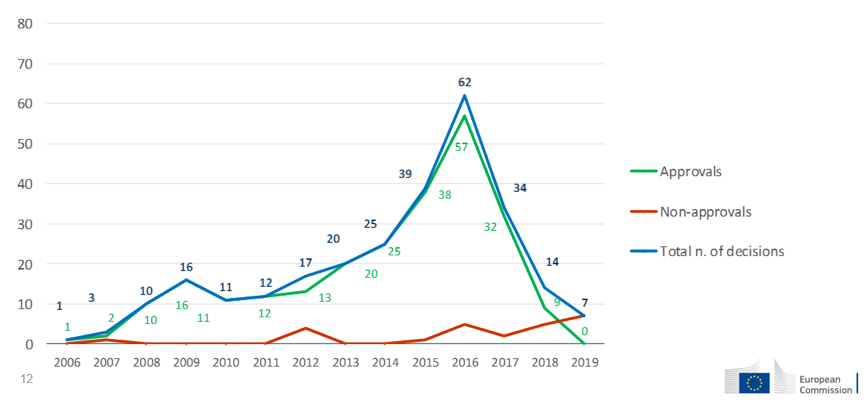
For biocidal products, a total of 14418 products have obtained authorization, consisting of national authorization, mutual recognition and simplified authorization. 767 authorizations came from German, followed by Spain (684), France (655) and Poland (642). The first unified authorization was made in 2018 and 21 unified authorizations have been granted thereafter, covering 107 products and 285 products are still in the progress.
Recent Development
As of 7 of June 2018, all ongoing and new procedures for active substance approvals have adopted the scientific criteria for the identification of Endocrine Disruptors established in 2017. ECHA will further compile and release procedural guidance for biocidal products, impurities in active substances and disinfection-by-production, as well as the names of non-active substances having indication of ED properties.
Second, the Union has taken effective actions to respond to the Covid-19 pandemic:
- Discussing and clarifying with Member States possibilities for granting emergency permits under Article 55(1) or adopting national measures to increase supply in disinfectants;
- Article 95 list of the BPR is a register of companies that have been approved as suppliers of specific biocidal active substances. The list can be expanded by ‘new suppliers’ of disinfectants through a letter of access from a listed company. Article 95 suppliers can procure disinfectant products from non-listed companies through technical equivalence determination and propan-1-ol and propan-2-ol can be determined through accelerated process.
- Input to the European Centre for Disease Prevention and Control (ECDC) in view of publication of guidance on the use of disinfectants
