Chinese New Food Contact Materials: First Quarter Announcements Analysis
Article 37 of Food Safety Law of the People’s Republic of China establishes that the use of new raw materials for food production, or the production of new food additives and new food-related products must be submitted to health administrative department of the State Council for safety assessment of the related products. In other words, if a substance is not listed in the positive list (such as the GB 9685-2016 positive list for additives used in food contact materials and articles) or in the relevant announcements, an application for the new substance is required. This article mainly analyzes and summarizes the new food contact materials announcement in the first quarter of 2018.
In the first quarter of 2018, only one announcement (announcement No. 3 of 2018) was issued and a total of six new substances were approved for use in food contact materials. The following section analyzes the profile, use, application type, and period for each of the six new substances.
1.Profiles
A brief description of the six new substances announced in the first quarter is shown in Table 1 below.
Table 1 Summary data of new substances announced in the first quarter
|
No. |
Chinese Name |
English Name |
CAS No. |
|
1 |
富含间戊二烯的C3-6石油馏分的均聚物及与以下一种或多种单体的共聚物:异丁烯、苯乙烯和α-甲基苯乙烯 |
Homopolymers of distillates (petroleum), C3-6, piperylene-rich (C5) and copolymers with one or more of the following: isobutylene (C4), styrene, and alpha-methylstyrene |
—— |
|
2 |
3-氨丙基三乙氧硅烷 |
3-Aminopropyltriethoxysilane |
919-30-2 |
|
3 |
己二酸与间苯二甲酸,顺丁烯二酸酐,2-甲基-1,3-丙二醇,2,2-二羟甲基丁醇和2,6-萘二甲酸二甲酯的聚合物 |
Polymer of adipic acid with isophthalic acid, maleic anhydride, 2-methyl-1,3-propanediol, 2,2-dihydroxymethyl butanol and dimethyl-2,6-naphthalene dicarboxyla |
—— |
|
4 |
间苯二甲酸与顺丁烯二酸酐,邻苯二甲酸酐, 磷酸,2,2-二羟甲基丁醇和2-甲基-1,3-丙二醇的聚合物 |
Polymer of isophthalic acid, maleic anhydride, phthalic anhydride, phosphoric acid, trimethylol propane and 2-methyl-1,3-propanediol |
—— |
|
5 |
间苯二甲酸, 对苯二甲酸,己二酸, 2,2-二羟甲基丁醇和2-甲基-1,3-丙二醇和乙二醇的聚合物 |
Polymer of isophthalic acid and terephthalic acid, adipic acid, trimethylol propane, 2-methyl-1,3-propanediol and ethylene glycol |
—— |
|
6 |
聚氯乙烯 |
Polyvinyl chloride |
9002-86-2 |
2. Uses
As shown in Figure 1, among the six new substances announced in the first quarter, one substance (No.1 substance) is applied to adhesives, and one substance (No.2 substance) is applied to polyamide (PA) plastics. The remaining four substances are used in paintings and coatings.
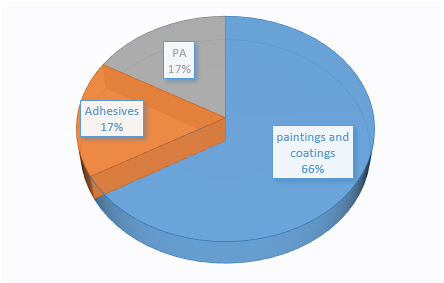
Figure 1 Uses of six new substances announced in the first quarter
3. Application types
As shown in Figure 2, among the six new substances announced in the first quarter, one substance (No.1 substance) belongs to the “new additive” portion, two substances (No.2 and No.6) belong to the “expanded scope or amount of use”, and the remaining three substances belong to the “new resin” category. The two substances that expand the scope or the amount of use can also be subdivided. The former (No.2) is sub-classified as an additive that expands the scope or amount of use, while the latter (No.6) may be sub-classified as a resin that expands the scope or amount of use.
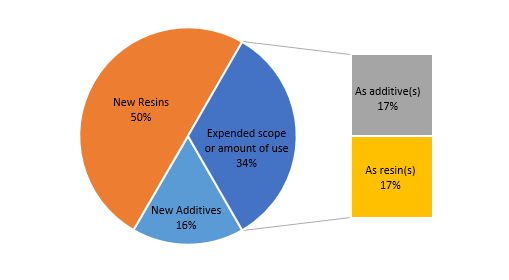
Figure 2 Application types of new substances announced in the first quarter
4. Periods
As shown in Table 2, among the six new substances announced in the first quarter, the total application period (from the time of acceptance of the application to the final announcement) may take as long as 3 years and 9 months. The shortest period can take 1 year only. We also know that the period that runs from the acceptance of the new substance to the public review is generally longer than the period that runs from public review to the final announcement. The reason for this is that during the first stage (application acceptance to public review), it is necessary to go through the careful expert evaluation, enterprise defense, and/or submission of additional supporting data to the risk assessment. Once the risk assessment is approved, it will be subject to further public review. Thus, the time required for that stage is relatively longer. After the substance is again submitted to public review, if the public has no objections on the grounds of safety or other circumstances, then the substance will be officially announced within a period six months to one year.
Table 2 Periods of new substances announced in the first quarter
|
Substance |
Period from acceptance to public review |
Period from public review to announcement |
Overall Application period |
|
Homopolymers of distillates (petroleum), C3-6, piperylene-rich (C5) and copolymers with one or more of the following: isobutylene (C4), styrene, and alpha-methylstyrene |
3 years and 3 months |
6 months |
3 years and 9 months |
|
3-Aminopropyltriethoxysilane |
8 months |
9 months |
1year and 5 months |
|
Polymer of adipic acid with isophthalic acid, maleic anhydride, 2-methyl-1,3-propanediol, 2,2-dihydroxymethyl butanol and dimethyl-2,6-naphthalene dicarboxyla |
11 months |
6 months |
1year and 4 months |
|
Polymer of isophthalic acid, maleic anhydride, phthalic anhydride, phosphoric acid, trimethylol propane and 2-methyl-1,3-propanediol |
11 months |
6 months |
1 year and 4 months |
|
Polymer of isophthalic acid and terephthalic acid, adipic acid, trimethylol propane, 2-methyl-1,3-propanediol and ethylene glycol |
11 months |
6 months |
1 year and 4 months |
|
Polyvinyl chloride |
6 months |
6 months |
1 year |
Brief summary:Following Article 5 of GB4806.1-2016 National Standards for Food Safety-General Safety Requirements for Food Contact Materials and Articles, on the principle of compliance: Raw materials, additives of and actual food contact materials and articles should comply with food safety standards and related announcements. Therefore, the six new substances announced in the first quarter of 2018 have the same regulatory effect as the substances in the positive list, and as such they are allowed to be used in food contact materials and articles as long as they comply with the corresponding restrictions mentioned in the announcement. Consequently, in addition to complying with laws and regulations, companies should also closely follow new food contact material announcements to avoid unnecessary compliance work.
