EU Amends Cosmetic Ingredient Use Requirements: 14 Newly Prohibited Ingredients and 2 Newly Restricted Ingredients
Early this year, the EU launched a public consultation on a draft Commission Regulation concerning using CMR substances (carcinogenic, mutagenic or toxic for reproduction) in cosmetics. On September 16, 2022, the EU introduced the finalized version of this Regulation. According to the Regulation, the adopted amendments will apply to all Member States from December 17, 2022.
EU to Restrict the Use of Methyl-N-Methyl Anthranilate
On February 1, 2022, the EU released an amendment to Regulation (EC) No 1223/2009 (Cosmetics Regulation), in which Methyl-N-methyl anthranilate (M-N-MA) (CAS No 85-91-6) was newly included in the restricted ingredients list with use restriction:
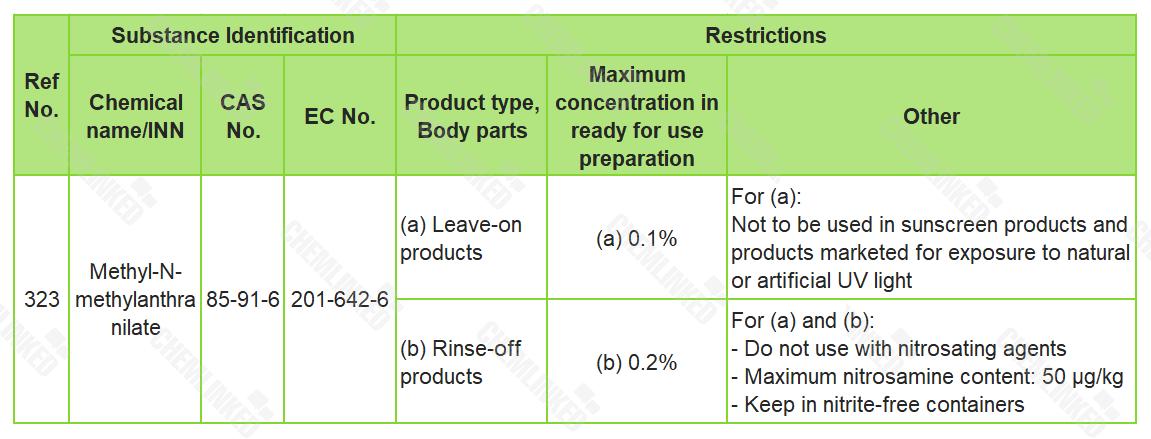 The amendment is scheduled to come into effect from February 21, 2022. Products containing M-N-MA and not complying with these conditions shall not be placed on the Union market from August 21, 2022 and not be made available on the Union market from November 21, 2022.
The amendment is scheduled to come into effect from February 21, 2022. Products containing M-N-MA and not complying with these conditions shall not be placed on the Union market from August 21, 2022 and not be made available on the Union market from November 21, 2022.
EU to Adopt Amendments Concerning CMR Substances
[Read more at ChemLinked]
Reference Links
- Cosmetics Regulation: EU Amends the Use Requirements for 2 UV Filters
- Free Webinar: Similarities and Differences Between ASEAN and European Cosmetic Regulations
Contact Us
We are devoted to providing professional global market access consultations and customized regulatory compliance services for cosmetic companies. If you need any help with cosmetic ingredient requirements in EU, please feel free to contact us.
Tel: +86-571-87103817
Email: customer@reach24h.com
News Source: ChemLinked

Market | Chemical | Food | Cosmetic | BaiPharm | Agrochemical
REACH24H Consulting Group launched ChemLinked in 2012 as a leading service provider of comprehensive regulatory information and compliance solutions, meeting the growing demand for clear and concise regulatory advice and market intelligence in Asia, especially China.
You can register for a membership to read the latest news limitlessly every day on ChemLinked.
