SFDA Finalized the First Batch of Existing Cosmetic Ingredients in China
Cosmetic ingredients that have been included in the Inventory of Existing Cosmetic Ingredients in China (IECIC) are exempted from registration with China’s SFDA. Last year, three draft batches totaling 3667 ingredients were released by the SFDA for public comment:
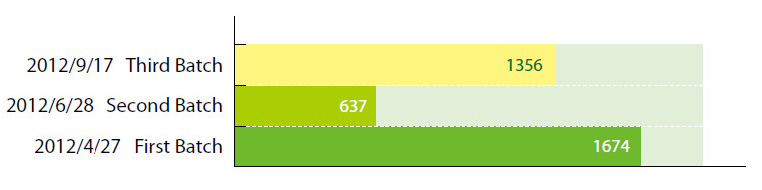
Recently, the first batch of SFDA sanctioned cosmetic ingredients was finalized and further authorizations are expected in the following months. There are 1674 ingredients listed in the final first batch.
The table below shows the structure of the IECIC:
| No. | Chinese Name | INCI Name | Maximum Quantity in the
Approved Cosmetic Products (%) |
| 1 | (动物)胎盘蛋白质 | PLACENTAL PROTEIN | 0.0007 |
| 2 | (日用)香精 | FRAGRANCE | 1 |
| 1674 | 组氨酸 HCl | HISTIDINE HCL | 0.01 |
Note: Maximum quantity refers to the maximum amount of an ingredient in the cosmetic products that have already been approved but do not mean the maximum concentration permitted in cosmetic products.
The inventory also contains restricted substances stipulated in the Hygienic Standard for Cosmetics (2007). Under this regulation manufacturers need to comply with the corresponding restrictions and requirements, for example
| Ingredient | Maximum Quantity in the Approved Cosmetic Products (%) | Specified limit of use in Hygienic Standard for Cosmetics (2007) | |
| Maximum concentration permitted in cosmetic products (%) | Other restrictions and requirements | ||
| 2,6-DIHYDROXYETHYLAMINOTOLUENE | 0.236 | 2 | In combination with oxidizing dye, the maximum use
concentration is 1.0% |
Please note this article originates from the chemlinked, for more information you may refer to www.chemlinked.com.
