Expert Insights: Analysis of EU Pesticide Renewal Programme -Case Study of Prothioconazole
The European Union is a region with a stable demand for pesticides. According to Eurostat, from 2011 to 2018, the sales of pesticides in the European Union remained at a consistent level of about 360 million kilograms per year. In addition, the Member States of the European Union, such as France, Spain, Italy and Germany, are in the leading position in the world in terms of both the level of agricultural development and the scale of agriculture. It can be said that the pesticide in the EU still has great market potential and EU is a good choice for strategic layout and development for pesticide manufactures.
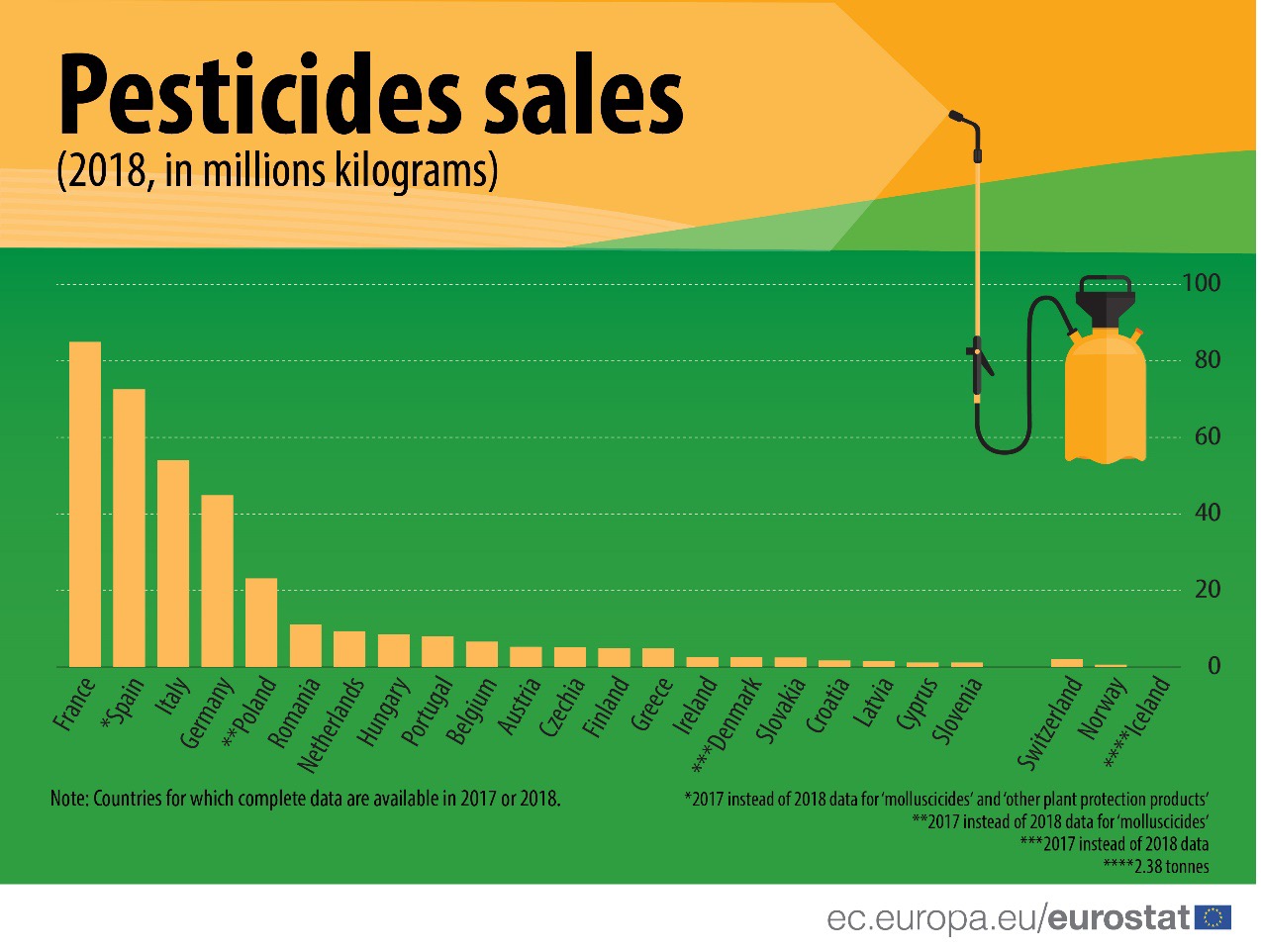 (Source: Eurostat)
(Source: Eurostat)
How can pesticide companies enter the EU market? The most cost-effective way is to apply for the technical equivalence assessment for the technical material, i.e. TE assessment. The TE assessment relatively has a short evaluation workflow and the cost is low. Generally, companies only need to spend less than ten thousand dollars to get the approval letter within a few months or one year, and then their products can enter the EU market.
TE assessment is a stepping stone for enterprises to enter the EU market, but when the door is opened, TE is not enough for enterprises to better carry out market layout. According to the EU pesticide regulations, the registration of pesticide formulations requires two sets of data, one for technical material and the other one for the formulation. The manufacturers generally prefer to purchase the technical material from owners who have a full set of application data to support their pesticide registration. Due to the fact that lack of registration data, TE source owners cannot support the registration of downstream manufactures, so there will be certain restrictions on sales. Generally, TE sources can only be sold as an alternative source to those who have the ability to complete the registration of pesticides by their own. Sales profits will also be limited. If manufactures of TE sources hope to take greater initiative in the EU market, join in the pesticide renewal programme will be a solution for them.
After participating in the renewal programme, the technical material approved during the renewal will become a reference source, which can not only be legally sold in the EU, but also have greater autonomy in sales because of the full set of technical material data. In the subsequent trade, customers can be freely selected, and it will be relatively easy to obtain customers than other sources, and higher profits can be obtained in sales.
In addition, participating in the renewal can keep abreast of the situation of the renewal at any time, avoid renewal applicants artificially set up technical barriers to trade through ways such as specification revision, and become a rule maker rather than just a participant in the game.
We all know that the management system of pesticides in the European Union is very strict, and the requirements for pesticides are very high. Therefore, if the products are officially recognized through the EU renewal and becomes a reference source in the EU market, it is a high recognition of product quality and a high-quality plus for the enterprises to carry out brand promotion.
Based on the above reasons, although the cost of pesticide renewal is relatively high and the cycle is relatively long, this investment is necessary for leading domestic pesticide manufacturers.
EU pesticide renewal programme
- Introduction to Renewal programme
According to Regulation (EU) 1107/2009, the EU implements a periodic renewal system for pesticides. In recent years, the renewal regulations have changed frequently. The original Regulation (EU) No 844/2012 has went through three major updates, and is now replaced by Regulation (EU) 2020/1740. The new regulation will apply to active substances for which the approval date ends on or after 27 March 2024 (excluding substances that expire after 27 March 2024 as a result of an extension of the expiry date). Active substances expiring before 27 March 2024 remain subject to Regulation (EU) No 844/2012.
According to the renewal regulations, the validity period of the approval of pesticide active substances in the European Union is generally 10 years (excluding low risk pesticides and pesticides candidates for substitution). Before the expiration of the approval period of an active substance, it is necessary to start the renewal process in order to complete the evaluation before its expiration, realizing the smooth renewal of the approval period. At present, if pesticide enterprises plan to support the renewal, a full set of data meet the data requirements of Regulation (EU) 283/2013 and Regulation (EU) 284/2013 is required to be submitted to the European Union at least 3 years before the expiry date of the active substance approval. The data requirements are the same as the current data requirements for new substance approvals.
Why would an applicant need to submit new data when the data requirements are the same for renewal and initial approval? Prothioconazole will be taken as a case study to clarify.
According to the renewal application data submitted by Bayer in 2015, the renwal data file included new data in product chemistry, analytical methods, toxicology, ecotoxicology, environmental fate, and residue. The main reasons for submitting new data are as follows:
1) Changes in legislation. Prothioconazole was approved in 2008 in accordance with Directive 91/414/EEC, the predecessor of the current EU Pesticide Regulation, however the renewal was required in accordance with Regulation (EC) No 1107/2009. Due to the change of the Regulation, the data requirements also changed. The renewal proponent will then need to submit new data to meet the new requirements.
2) Change of guidance documents. For example, in the part of analytical methods, the referenced guidance documents SANCO/3030/99, SANCO/3029/99 and SANCO/825/00 have been updated in the EU in recent years, and applicants need to carry out tests and provide corresponding data in accordance with the requirements of the new guidelines.
3) With the progress of science and technology, new test methods are available to perform the tests for which that could not be performed before.
4) Typical formulation change. In this renewal, in addition to the same typical preparation as the original substance approval, Bayer has added a new typical preparation, and the two preparations are different in composition and use, so new data need to be supplemented.
- Revision of Renewal Rules
The change of renewal regulation is also accompanied by a series of changes in implementation requirements. Based on years of experience on EU regulations, the author believes that the current renewal regulations are more friendly to applicants due to the reasons below.
1) Study notification requirements. According to the latest guidance document issued by EFSA in March this year, after March 27, 2021, the applicant is suggested to notify EFSA at least five months before submitting the application about the studies planed to be carried out to support the renewal. Studies submitted directly without notification will have a risk of rejection during evaluation. Although the process become more cumbersome, it avoids the risk of applicants running unnecessary or unsuitable tests.
2) Changes in dossier format. The original dossier format for EU pesticide registration is CADDY, which is very difficult to make. In addition to experts in registration, it is also necessary to get involved in IT experts, or turn to a special software company to help. Under the new regulations, the dossier format is changed to IUCLID, which is the same format as for other EU regulations such as REACH and BPR, and the difficulty of dossier compilation is greatly reduced.
3) Information transparency. According to the Transparency Regulation, the whole process of renewal will be more open and transparent. The dossiers submitted by applicants, as well as official evaluation records of authorities will be made public in OpenEFSA in a timely manner, and stakeholders will have easier access to these information than in the past. In addition, EFSA has launched Connect.EFSA in order to strengthen the good communication between the applicant and the assessor in the evaluation process and promote the smooth progress of the renewal work.
Analysis of EU Renewal Strategy
Participating in the EU pesticide renewal is a big decision for pesticide enterprises, and careful planning is needed. What should we pay attention to during the decision-making stage? The author believes that three “Rights” need to be considered, that is right time, right place and right substance. The three “Rights” will be analyzed in detail accordingly.
- Right Time
In the European Union, after the approval of a new active substance, other technical manufacturers can only enter the market through TE assessment for most of the time. The European Union has a special work plan for the pesticide renewal. If pesticide enterprises want to participate in the renewal, they must join in accordance with the official renewal schedule. At present, there are six Active Ingredient Renewal Programmes (AIR-Programme). Each programme is created generally based on the active substance approval period.
| AIR | Approval Expiry Date | Substance Number | Progress |
| AIR-1 | Directive 91/414/EEC | 7 | Finished |
| AIR-2 | Directive 91/414/EEC | 31 | Finished |
| AIR-3 | 2013.1.1-2018.12.31 | 150 | In progress |
| AIR-4 | 2019.1.1-2021.12.31 | 215 | In progress |
| AIR-5 | 2022.1.1-2024.12.31 | 66 | In progress |
| AIR-6 | 2025.3.31-2028.12.27 | 28 | In plan |
Taking Prothioconazole as an example, Bayer submitted an application for substance approval of Prothioconazole to the United Kingdom on March 25, 2002, which was approved on August 1, 2008, with a validity period of 10 years and a deadline of July 31, 2018. Prothioconazole is a substance in the AIR-3 programme according to its expiry date.
According to the renewal regulations, the applicant for renewal must submit the application for renewal three years before the expiry date, that is, before July 31, 2015. According to the relevant work plan, Bayer is the only supporter of Prothioconazole renewal, and they submitted an application for renewal on July 28, 2015.
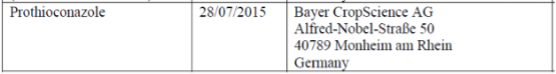
(Source: EC working document)
Anyone miss the deadline for renewal submission will not be allowed to apply. After the deadline, anyone wish to participate in the renewal can only negotiate with the existing renewal applicants to form a task force. At this point, the decision is in the hands of the existing renewal applicants. Therefore, enterprises willing to participate in the renewal must pay attention to the approval period of the target active substance and the working document of AIR programmes, making sure to submit the application for renewal to the authorities in time.
According to related regulations, the review period of the technical material takes nearly three years, but in recent years, most of the reviews have been postponed. For example, although Bayer has submitted the application for the renewal of Prothioconazole in 2015, the evaluation is still not completed, and the expiration date is extended to July 31, 2022 now. According to EFSA review record, it suspended the review from October 22, 2018 to March 15, 2020 to allow the applicant preparing additional information to support the application. At present, the review was suspended again on October 16, 2020 due to the need for more data for endocrine disruption assessment. The review is expected to be launched again after April 15, 2023. Therefore, the validity period of prothioconazole should continue to be extended.
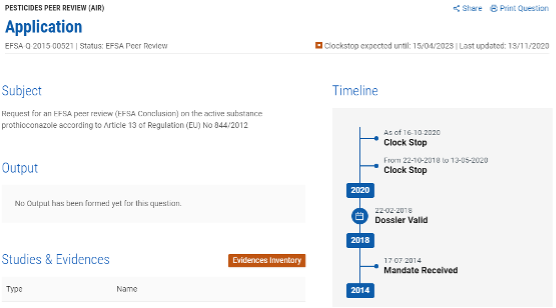
(Source: EFSA website)
After the renewal of the active substance is completed, the renewal of the plant protection products will follow. Taking prothioconazole as an example, the number of prothioconazole formulations registered and the number of registration certification holders is relatively large. The countries have the most registered products are the United Kingdom and France, with more than 100 products registered. In addition, Germany, Belgium, Italy, Poland, and Sweden also have dozens of registrations. In addition to Bayer, there are dozens of registration certification holders including Clayton Plant Protection (UK) Limited, SAGA SAS, GRITCHE, Life Scientific Limited, etc. Due to the postponement of the active substance renewal, the registration validity period of the preparations also extended accordingly. For prothioconazole, the current registration validity period of preparations in various member states has basically been extended to after 2022 or even later.
- Right Place
Although the active substance renewal is carried out at the EU level, there will be an rapporteur member state (RMS) to perform the first step of the dossier review, issue an RAR (Renewal Assessment Report), and then submit it to EFSA for peer review and issue the final risk assessment report.
The choice of RMS has a particularly large impact on the overall assessment. At present, due to the impact of Brexit, the workload of Member States is very heavy, which is also an important reason for the postponement of the EU\’s renewal programmes in recent years. In addition, the evaluation capability of EU Member States varies greatly, and not every country has the ability to conduct independent evaluation. Czech Republic perform the assessment of different data such as product chemistry, toxicology, etc. by different departments, and the applicant needs to contact multiple PMs. As a result, the applicant\’s active and effective communication with RMS during the review process will be affected. Besides, different Member States have different evaluation styles, for example, Belgium has a relatively more strict requirements for revaluation. Finally, Member States charge differently for the assessment. For example, for the renewal of active substance, Sweden will charge up to 500,000 euros for the evaluation fee, while Germany will charge between 127,000 and 251,000 euros.
According to the regulations, the RMS of renewal is designated by the European Union rather than nominating by the applicant, so it is very important to know the style of RMS. Being familiar with the \”temperament\” of RMS will help with the smoother communication during the review.
- Right Substance
Whether the active substance will be banned is a popular topic that the renewal applicants will pay attention to. Choosing the right substance to support can prevent enterprises from flogging a dead horse. For pesticide registration, having a safe use has been a determining factor in the approval decision. But the European Union is not a completely risk-oriented region, and under certain circumstances, the hazard of the active substance also plays a decisive role in whether the substance can be approved. Based on the risk of use as well as the substance hazard, the European Union has banned many active substance of pesticides, which also makes the European Union a very difficult area for pesticide registration.
According to Regulation (EC) No 1107/2009, there is a cut off criteria for the approval of an active substance. If this criterion is met, the EU will not conduct a risk assessment on the active substance but directly prohibit its use. The cut off criteria are as follows:
1) For human health, a substance is prohibited if it is classified as a CMR substance or an endocrine disruptor;
2) Environmentally, a substance is prohibited if it is regarded as PBT, POP, or vPvB.
For example, because prothioconazole and its metabolites may have potential endocrine disrupting effects, the applicant is still supplementing additional information to exclude its endocrine disrupting hazards.
