Turkey KKDIK
What is KKDIK?
On 23 June 2017, the Ministry of Environment and Urbanization (MoEU) of Turkey published its KKDIK regulation which is very similar to EU REACH regulation, aims to regulate the chemicals’ safe use in the Turkey market to ensure a high level of protection of human health and environment.
The KKDIK regulation came into force on 23 December 2017. This repealed and replaced the following three existing laws:
- The Inventory and Control of Chemicals(CICR-27092)
- The Preparation and Distribution of Safety Datasheets for Hazardous Materials and Products (29204)
- The Restrictions Relating to the Production, Supply to the Market and Use of Certain Hazardous Materials, Products and Goods (27092)
The Regulation Scope of KKDIK
Similar to EU REACH regulation, the KKDIK regulation applies to chemical substances either on their own, in mixtures or in articles. KKDIK requires companies to register all substances manufactured in Turkey or imported to Turkey with volume balance to or above 1 tonnage per year to MoEU and provide safety data sheet (SDS) as well. Besides, companies should fulfill obligations of Evaluation, Authorization and Restriction.
Exemptions
KKDIK registration shall not apply to the substances and mixtures below:
- Radioactive substances and mixtures;
- Substances, on their own, in a mixture or in an article, which are subject to customs supervision, provided that they do not undergo any treatment or processing, and which are in temporary storage, or in a free zone or free warehouse with a view to re- exportation, or in transit;
- Non-isolated intermediates;
- The carriage of hazardous substances and hazardous mixtures by rail, road, inland waterway, sea or air;
- Wastes in the scope of the Bylaw on Waste Management published in the Official Gazette dated 02/04/2015 and numbered 29314 and Bylaw on Radioactive Waste Management published in the Official Gazette dated 09/03/2013 and numbered 28882.
- Substances and mixtures which are manufactured or imported for the purpose of defense;
- Medicinal products;
- Food and feeds;
- Veterinary products;
- Substances included in Annex 4 and 5
Obligations of Companies
Registration
1. Who should fulfill registration obligation?
- Manufacturer
- Importer
- Only Representative (OR)
2. Registration Type
For the substances that are manufactured or imported to Turkish market either on their own or in mixtures or in articles with an intended release with volume balance to or above 1 tonnage per year, KKDIK registration includes two stages: pre-registration and registration respectively.
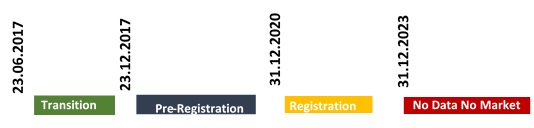
All pre-registrations should be submitted before 31 December 2020 and all registrations must be completed between 31 December 2020 and 31 December 2023.
Unlike EU REACH regulation, registration deadline under KKDIK is irrelevant with tonnage band. After 31.12.2023, substances manufactured in Turkey or imported into Turkey with volume balance to or above 1 tonnage per year couldn’t be placed on Turkish market unless it is registered to MoEU.
Pre-registrations and registrations should be submitted through KKS tool.
3. Important Time Node
Authorization
Substances on Annex XIV of KKDIK can‘t be manufactured, imported or used unless an authorization has been granted. Authority will start to identity SVHC and screen the list of candidates for Authorization after 31 December 2023.
Restriction
It must comply with restriction conditions set out in Annex XVII of KKDIK when certain chemicals manufactured, placed on the market and used. This Annex is in line with Annex XVII of EU REACH.
SDS
The supplier of a substance or a mixture shall provide the recipient of the substance or mixture with a safety data sheet in Turkish for information communication in the supply chain when meet the following conditions:
a) Where a substance or a mixture meets the criteria for classification as hazardous in accordance with the Bylaw on Classification, Labelling and Packaging ofSubstances and Mixtures or
b) Where a substance is persistent, bio accumulative and toxic or very persistent and very bio accumulative in accordance with the criteria set out in Annex 13; or
c) Where a substance is included in the list established in accordance with Article 49 for reasons other than those referred to in points (a) and (b).
C&L (Classification&Labelling) Notification
In addition to above KKDIK regulation obligations, manufacturers or importers have to submit C&L Notification for substances that are classified as hazardous to the Turkish Competent Authority to form the C&L inventory according to Turkish CLP regulation (SEA).
Company should submit C&L Notification through KKS tool.
*It should be noted that, similar to EU REACH regulation. For manufacturers outside Turkey, they should appoint a qualified Only Representative (OR) within Turkey to comply with KKDIK compliance. In addition, KKDIK require certified people who have received the certification described in KKDIK Annex XVIII to conduct the work of dossier, CSR as well as SDS preparation. Therefore, non-turkish companies should pay particular attention to abilities and qualifications when they appoint OR within Turkey.
Our Services
- Only Representative
- KKDIK Compliance Analysis
- C&L Notification
- Pre-registration
- Registration Scheme Development and Implementation
- Testing Supervision
- Safety Data Sheet (SDS) and Label Preparation Compliance with KKDIK
- Training and Consultancy on KKDIK
- Competent Authority/ Expert Communication
Our Advantages
- Rich experience from years of compliance practice with EU REACH
- Technical expertise to ensure highly professional support
- Over 150 professionals with extensive academic and practical experience in chemistry, toxicology, and other related fields
- In-depth cooperation with various laboratories, provide professional and efficient testing proposal
- Long-term and stable local partners in Turkey, provide professional technical supporting, as well as solving language communication problems.
- Fast response to customer requests, strictly confidential customer information, keep customer information confidential.




