EU REACH
Overview
REACH is the European Regulation on chemicals and their safe use. REACH stands for Registration, Evaluation, Authorisation and Restriction of Chemicals. The REACH regulation came into force in 1 Jun 2007, establishing a uniform system for the control of chemicals manufactured or imported to the EU.
Following the introduction of REACH, chemical substances on their own or in mixtures, manufactured or imported to the EU in quantities at above 1 tonne a year must be registered with the European Chemicals Agency (ECHA). This obligation also applies in certain cases to substances in articles. Failure to register means the company is no longer allowed to manufacture the substance in or import it to the EU.
The core contents under EU REACH can summarized as follows:
• Four Obligations:
Registration, Evaluation, Notification/Authorisation, Restriction and e(SDS)
• Three Chemical Objects:
Substances (phase-in substance and non-phase-in substance), Mixture, Article
• Four Major Roles:
EU Manufacturers, EU Importers, Companies acting as Only Representatives, Downstream Users
• Three Administrative Tools:
REACH-IT for information communication, IUCLID for creating dossier, Chesar
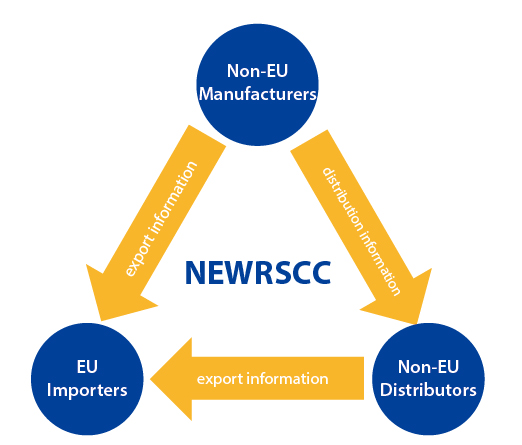
REACH Supply Chain Compliance System-NEWRSCC
NEWRSCC is the acronym for the NEW REACH Supply Chain Compliance System. Designed by REACH24H, it is an upgraded REACH compliance IT tool based on the RSCC system. Applying this specially-designed IT solution, NEWRSCC help manage related REACH compliance data and acquire the tonnage coverage certificates on the supply chain with a higher level of security. This upgraded tool has a friendly user interface, a compact operation procedure and a strict and confidential mechanism. It assures the prompt communication of different trade roles and satisfies REACH compliance requests through the supply chain.
Other Obligations under REACH
If a given substance constitutes a risk to human health or the environment, ECHA and the Member States will evaluate the information submitted by companies, and even include the substance in the Authorisation List or Restriction List. Manufacturers, importers or downstream users of a substance on the Authorisation List can apply for authorisation. Restrictions may limit or ban the manufacturing and placing on the market or use of a substance.

How to Comply?
FAQ
Q: If a non-Phase-in substance is used as intermediate, should we submit an inquiry before registration?
A: The inquiry is independent from the use of a substance. No matter whether a non-Phase-in substance is used as intermediate or not, it should submit an inquiry before registration. Once the inquiry is answered by ECHA, for intermediates below 1000t/a, we could just use the free data and submit the available data for registration.
Q: If a non-EEA manufacture submits a registration under one OR, could he appoint another OR for authorisation?
A: Regardless whether the substance in Annex XIV has been registered or if an OR has participated in the registration, the non-EEA manufacturer may appoint another OR to apply for authorisation. The ORs used for the registration and authorisation processes may be different entities.
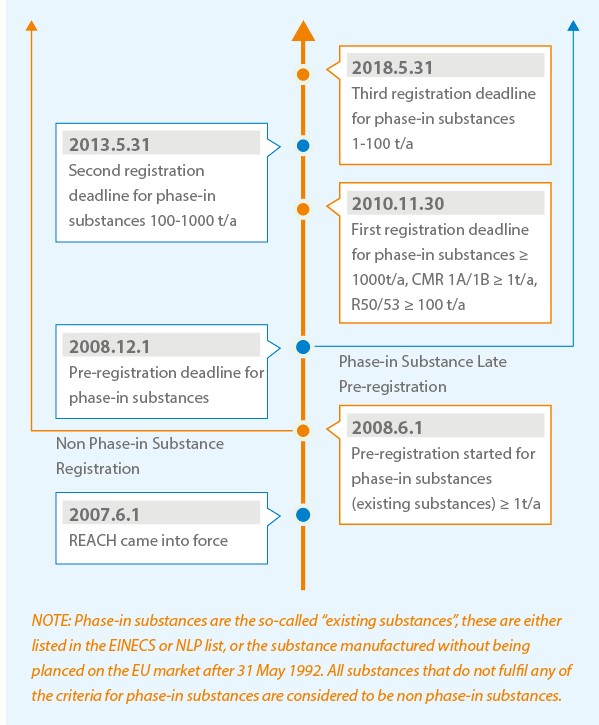
Registration Timeline
Applicable Scope
Registration shall apply to any of the following conditions under EU REACH:
• Substance as such, Manufacture / Importation > 1 t/a;
• Substance in a mixture, Manufacture / Importation > 1 t/a;
• Substance intended to release in an article, Manufacture / Importation above a concentration of 0.1% (w/w) & > 1 t/a ;
Note: The release of a substance from an article is deliberately planned and has a specific function in the use of the article. This is frequently not the main but an accessory function of the object, such as air freshener.
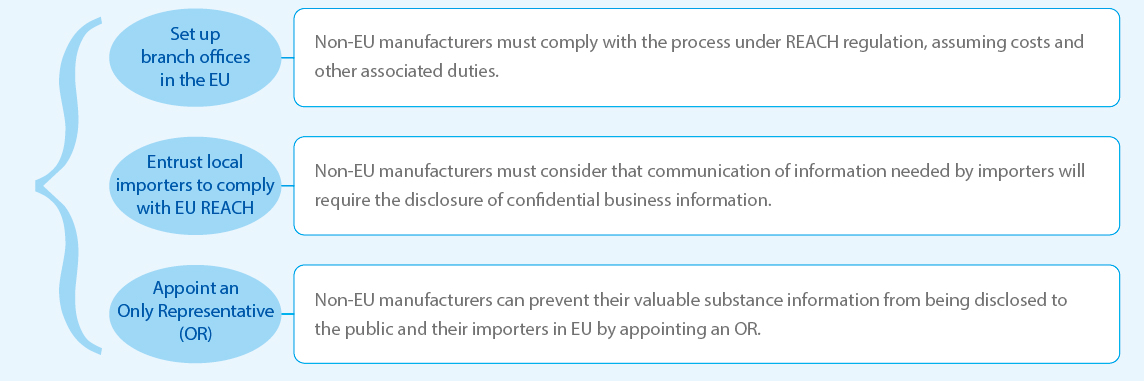
Registration Bodies
• Manufacturers and importers of substances, mixtures, article in EU;
• Non-EU companies can export to the European Union through two different routes under REACH: either via an importer who has registered the substance, or by appointing an Only Representative (OR).
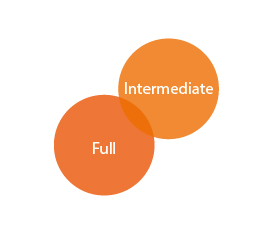
Registration Types
REACH covers different types of registrations: full registration (Article 10) and intermediate registration (Article 17 and 18).
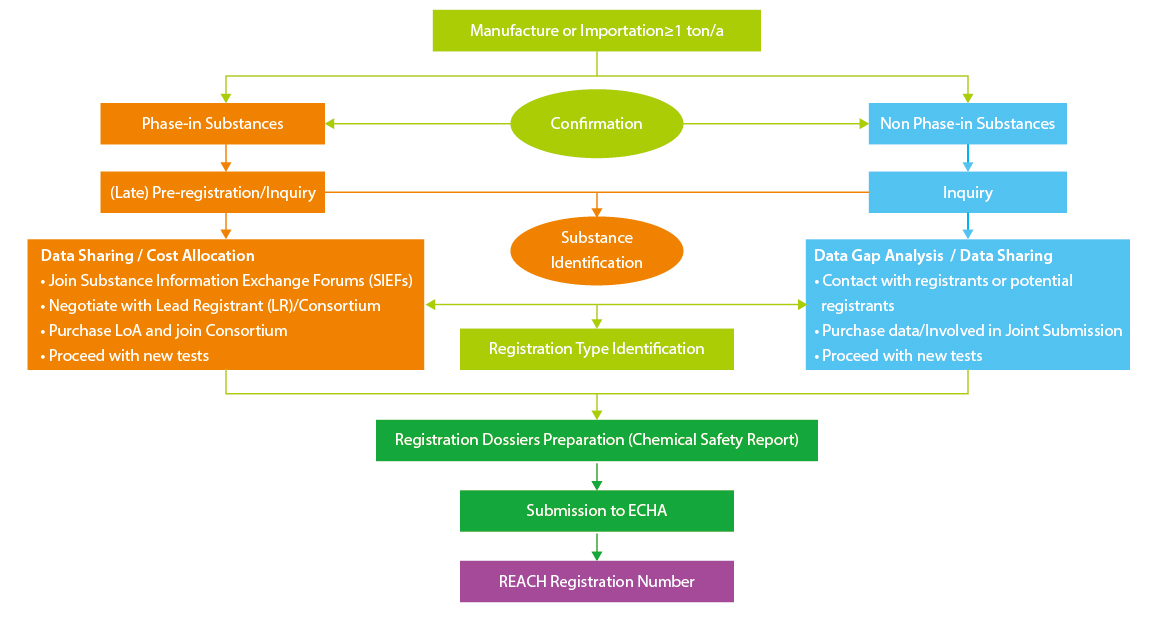
Regular Registration Procedure
Our Services
- Only Representative (OR) Service
- Inquiry Service
- Registration Service
- Lead Registrant Service
- OR Exchange Service
- Third Party Technical Support
- Testing Supervision Service
- PPORD Notification Service
- Exemption Declaration Service
- SIEF Management
- Exposure Scenario (ES) Development
- Chemical Safety Assessment and Chemical Safety Report (CSR) Preparation
- Extended Safety Data Sheet (eSDS) Preparation
- Compliance Check of Qualified Suppliers
- REACH Supply Chain Compliance System-NEWRSCC
- SVHC Notification Service · Authorisation Application Service
- REACH Training Service · HSA Inspection Assistance Service
- Consortium Supporting Service
Why REACH24H?
Technical Strength
- Technical expertise to ensure highly professional support
- Over 150 professionals with extensive academic and practical experience in chemistry, toxicology, and other related fields
- Close collaboration with certified test facilities
- Regular communications with ECHA and experts
Achievements
- Consulting services for PetroChina to obtain REACH Registration Number
- LR service experience on rubber, flavor & fragrance, chemical, pharmaceutical, and agrochemical industries, among others
- Strict quality control system to ensure 100% success rate:
3000+ EU REACH Registrations
30+ Lead Registrations




