REACH24H Achieves INCI Name for Innovative Cosmetic Exosomes, Unlocking Global Market Access
Hangzhou, China –March 26, 2025– REACH24H, serving as the exclusive agent, in collaboration with Beijing Guowei Biotechnology Co., Ltd., proudly announces that their innovative ingredient, Human Umbilical Wharton’s Jelly Mesenchymal Stem Cell Exosomes, has successfully cleared the rigorous evaluation by the International Cosmetic Ingredient Nomenclature (INC) Committee.
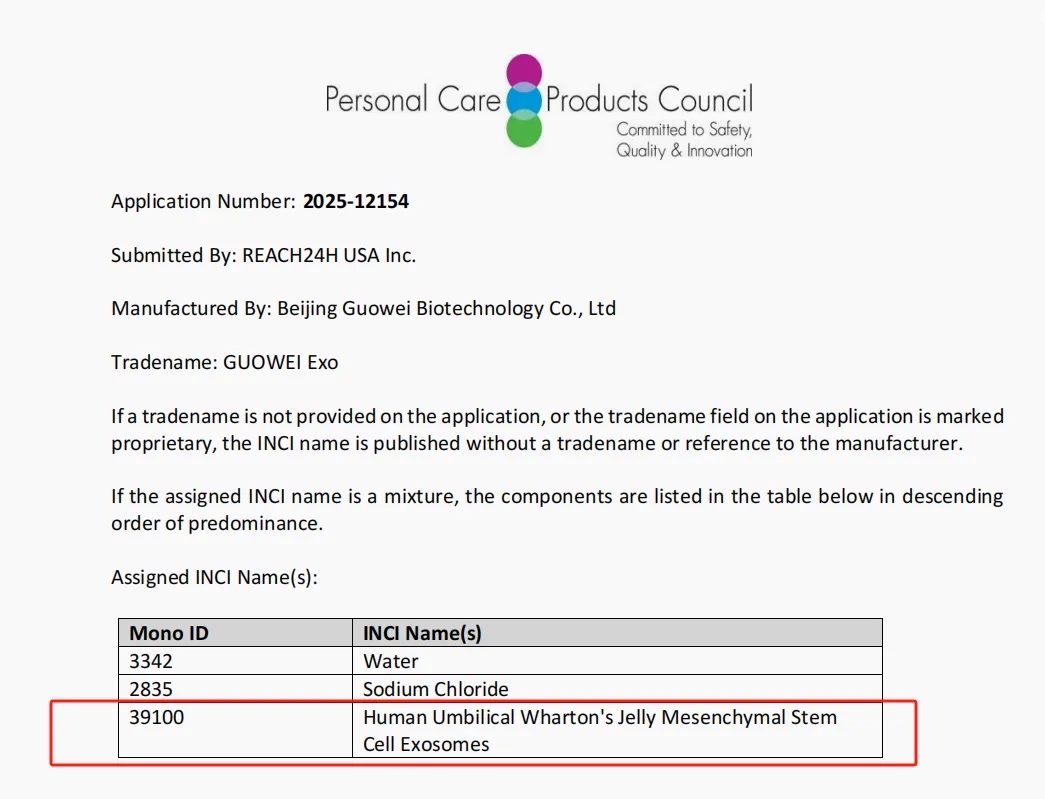
This milestone represents a pivotal advancement in the ingredient’s global regulatory compliance journey, as it is now officially recognized in the International Cosmetic Ingredient Dictionary and Handbook and included in the wINCI database.
INCI Name: A Passport for Global Market Expansion
The adoption of an INCI name provides a universally recognized identifier for cosmetic ingredients. This designation confirms that the ingredient meets the stringent requirements of key markets including the United States, the European Union, China, and others. In adherence with regulations such as the EU Cosmetic Regulation (No. 1223/2009), the INCI label mitigates trade risks associated with nomenclature discrepancies while simplifying the registration processes across diverse regions.
Innovative Science: Exosomes as Biological Nanocarriers
Exosomes, a specialized subgroup of extracellular vesicles measuring between 20 and 200 nanometers, contain a host of bioactive molecules—proteins, lipids, polysaccharides, and RNA—that are essential for cell-to-cell communication. Acting as biological nano messengers, these exosomes play a pivotal role in conveying signals between cells. The newly designated product, derived from human umbilical cord mesenchymal stem cells, exemplifies the forward-thinking application of human-sourced exosomes in enhancing anti-aging and regenerative repair technologies.
Global Compliance Expertise: One-Stop Cosmetic Compliance Solutions
Backed by an expert team of over 40 industry professionals, REACH24H offers a comprehensive, one-stop solution for navigating the complexities of global market registration. The team includes internationally certified toxicologists, EU-certified cosmetic safety assessors, risk evaluators, formulation scientists, regulatory analysts, and multilingual professionals. This depth of expertise not only accelerates market entry by streamlining regulatory processes but also supports research and development through access to extensive toxicological data and strategic patent navigation.
For inquiries or more information about cosmetic products and ingredients compliance, please contact customer@reach24h.com.


