Prothioconazole’s Promising Future: EU Renewal Assessment Finds No Evidence of Endocrine Disrupting Effects
Prothioconazole, a broad-spectrum triazolinthione class of fungicides developed by Bayer and launched in 2004, features such advantages as broad-spectrum fungicidal activity, good systemic activity, high protective, curative and eradicative activity, and long effective period, with prominent control effect on wheat head blight. However, like other triazole pesticides, the potential endocrine disrupting effect of prothioconazole and its metabolite prothioconazole-desthio has been of great concern globally and is the key item to be assessed in the pesticide registration process.
According to the recently disclosed result of the EU periodic renewal assessment of prothioconazole, for which Bayer is the lead applicant, it is initially concluded that prothioconazole does not meet the endocrine disruptor (ED) criteria.
History of the EU Renewal Assessment of Prothioconazole
The renewal assessment process began in February 2018, with the UK as the Rapporteur Member State (RMS) completing the initial draft renewal assessment report (dRAR). Due to the UK’s exit from the EU, Poland and France were respectively designated as the RMS and Co-RMS to continue the renewal assessment, and the dRAR was first updated in 2020 after peer review.
However, due to the formal implementation of the Guidance for the identification of endocrine disruptors in biocides and pesticides (hereinafter referred to as the ED Guidance) issued in 2018, the European Food Safety Authority (EFSA) required additional submission of the new data package for non-target organisms from prothioconazole applicants, triggering ED Clock Stop. The renewal assessment of prothioconazole resumed in 2022 after the applicant submitted new data, and a new dRAR was officially released in March 2023.
The deadline for public consultation on the prothioconazole dRAR is scheduled to end on May 29, 2023. EFSA plans to release the final risk assessment conclusion on October 30, 2023, based on the latest dRAR and the public consultation feedback.
Endocrine Disruption Assessment for Prothioconazole
Part 1 Guidance for the identification of endocrine disruptors in biocides and pesticides
The Guidance for the identification of endocrine disruptors in biocides and pesticides is made based on the World Health Organization/International Program on Chemical Safety (WHO/IPCS) definition, which requires the presence of endocrine activity and the identification of adverse effects in both human and non-target organisms in the environment. The most critical part is to establish a causal relationship between the endocrine-disrupting activity and the observed adversity, which necessitates a mechanism of action (MoA) analysis.

Based on the definition of endocrine disruptors, the Organisation for Economic Co-operation and Development (OECD) developed a Conceptual Framework for Testing and Assessment of Endocrine Disruptors, which is classified into five levels , with a focus on the estrogen, androgen, thyroid and steroidogenesis (EATS)-mediated activity.
Specific test or data requirements for each level are summarized below:
Level 1 consists of a thorough search and retrieval of existing data and the preliminary identification of endocrine disrupting effects of compounds using non-test methods such as (Q)SAR, read-across and molecular docking.
Levels 2 and 3 focus on studying the endocrine activity of compounds. Level 2 mainly involves in vitro assays, while Level 3 mainly involves in vivo assays. These levels are considered key additions to the OECD guidelines due to the limited number of test guidelines conforming to them.
Levels 4 and 5 focus on studying the adversity of endocrine disruption caused by compounds. The test contents are basically derived from established OECD guidelines (e.g., OECD TG 414, 416, etc.), which aim to identify the adversity associated with endocrine disruption in traditional tests as possible. The main difference between Level 4 and Level 5 is that Level 5 emphasizes the identification of adversity over the entire life cycle of the tested species (e.g., OPPTS 850.1500: Fish Life Cycle Toxicity).
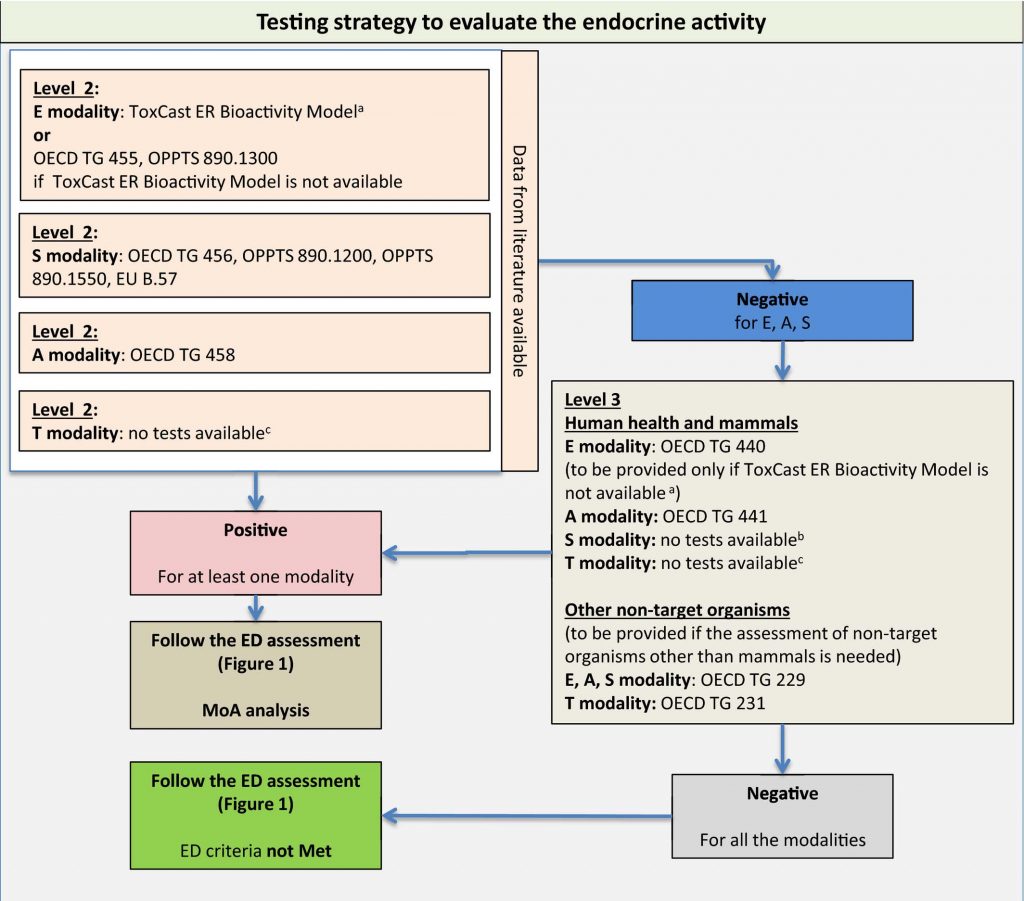
Testing strategy to evaluate the endocrine activity
(Source: Guidance for the identification of endocrine disruptors in biocides and pesticides)
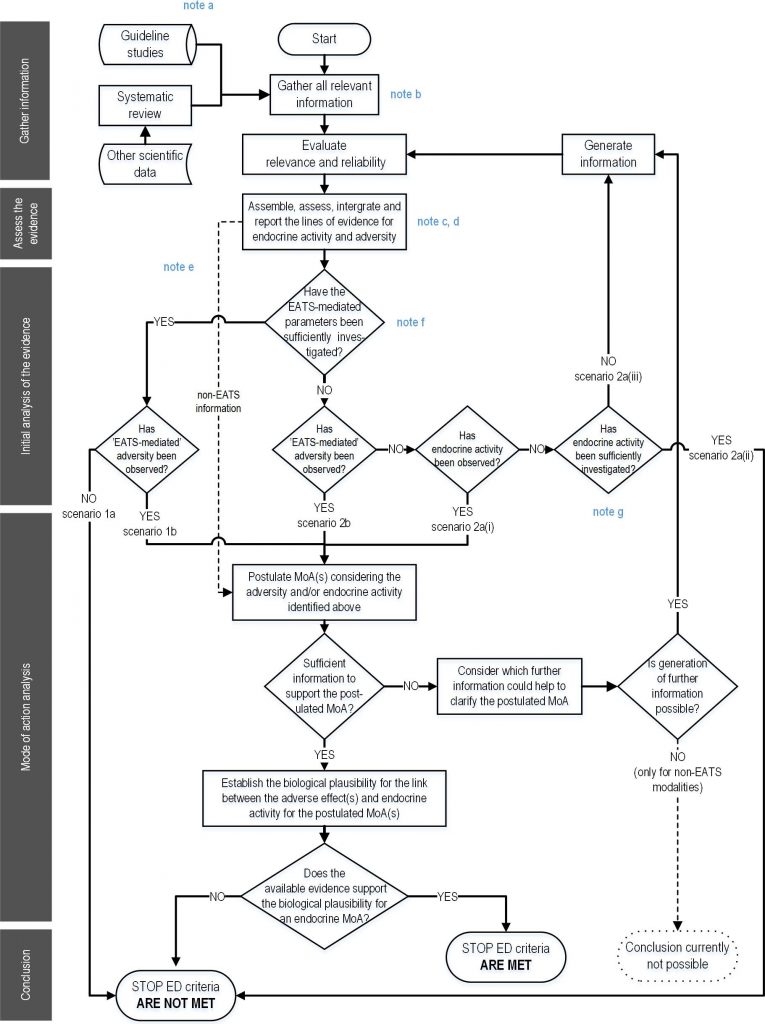
The test contents outlined in the ED Guidance are exactly based on the OECD Conceptual Framework. Applicants are required to generate, supplement, and organize a series of endocrine disruption data packages for humans and non-target organisms on target compounds, and carry out weighted analysis using the Weight of Evidence (WoE) approach. Based on the assessment flowchart (see below) outlined in the guidelines, a conclusion on whether the target compound falls under the category of an endocrine disruptor will be reached.
Flowchart illustrating the endocrine disruptor assessment strategy
(Source: Guidance for the identification of endocrine disruptors in biocides and pesticides)
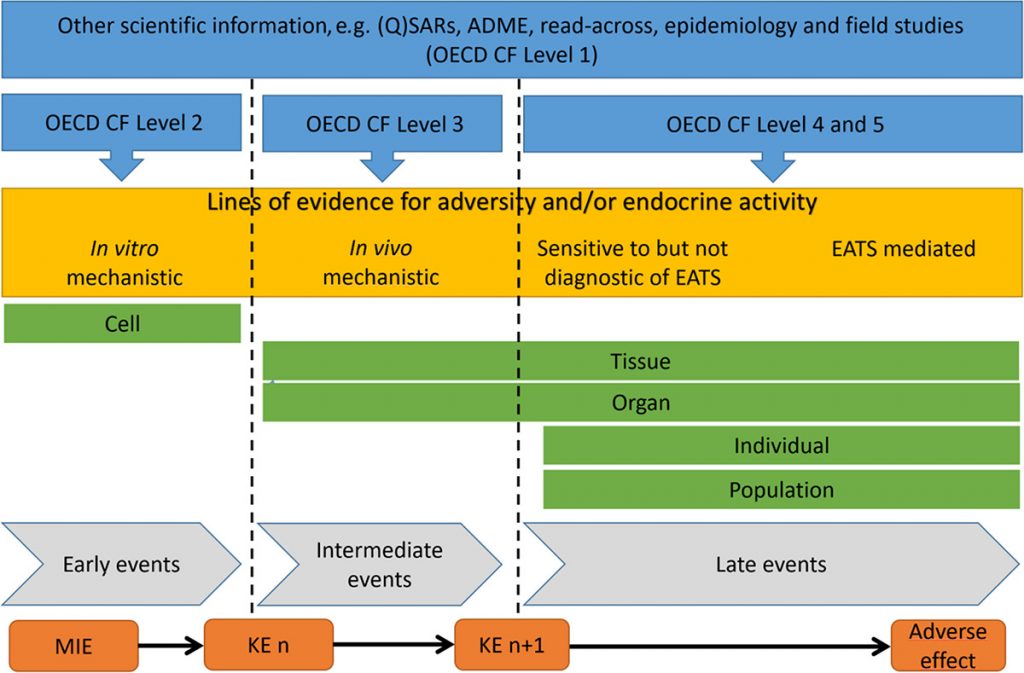
The challenge brought about by the ED Guidance lies in the requirement for further mode of action (MoA) analysis once a compound has been identified as having potential endocrine disruption activity and adversity, to establish the causal relationship between the activity and the adversity. Thanks to the maturity of adverse outcome pathway (AOP), the AOP framework has been introduced in the guidelines for the identification of endocrine disrupting effect of target compounds.
Adverse Outcome Pathway: Scheme illustrating how the lines of evidence can be organized to support the postulated mode of action
(Source: Guidance for the identification of endocrine disruptors in biocides and pesticides)
Part 2 Health toxicology assessment of prothioconazole for endocrine disruption
As the toxicity assessment of prothioconazole for endocrine disruption was completed in 2018 by the then RMS UK, the overall assessment process did not follow the ED Guidance, which was introduced later. Additionally, for the Clock Stop of the prothioconazole renewal assessment triggered by the endocrine disruption assessment, the assessment body deemed that, based on the existing data and assessment conclusions, no additional toxicologic data was required, therefore, the section concerning toxicity assessment of endocrine disruption is not updated in the new prothioconazole dRAR released recently.
Specifically, the data for the health toxicity assessment of prothioconazole for endocrine disruption were mainly based on existing toxicology information that meet the testing requirements of OECD Conceptual Framework Levels 4 and 5. Though two in vitro studies on endocrine disruption of prothioconazole were identified through literature search, it was finally confirmed by thorough WoE analysis that prothioconazole does not fall under the current EU definition of endocrine disruptors for humans.
Part 3 Ecotoxicity assessment of prothioconazole for endocrine disruption
The ecotoxicity assessment of prothioconazole for endocrine disruption was the focus of the ED Clock Stop for prothioconazole renewal assessment. Regarding the test data, existing relevant ecotoxicology data and a series of in vitro assay data from the US ToxCast database were used, and two new sets of OECD Conceptual Framework Level 3 test data, namely OECD TG 248 Xenopus Eleutheroembryonic Thyroid Assay (XETA) and OECD TG 229 Fish Short Term Reproduction Assay (FSTRA), were supplemented by the applicant. In addition, the applicant also conducted a systematic literature search and identified three studies on the endocrine disruption of prothioconazole out of 614 publicly available documents, which were then subjected to further comprehensive assessment.
Preliminarily assessment result of prothioconazole
The entire process for the assessment of prothioconazole for endocrine disruption followed the latest ED Guidance, and the conclusion was based on a thorough weight of evidence (WoE) analysis. The results showed prothioconazole generates no EATS-mediated endocrine disrupting adversity in wild mammals and no EATS-mediated endocrine disrupting activity in non-target aquatic organisms. Finally, it was concluded that prothioconazole does not fall under the current EU definition of endocrine disruptors for non-target organisms.
Based on these test results and further systematic analysis provided by the applicant, the EU RMS preliminarily concludes that prothioconazole does not meet the ED criteria.
A Promising Future for Prothioconazole
Prothioconazole is a star product among all broad-spectrum fungicides, and its development momentum is approaching the leading position of azoxystrobin. With the acquisition of registration certificates by numerous pesticide manufacturers in recent years and the further unlocking of production capacity, it can be expected that the sales and output of prothioconazole will increase simultaneously. The release of the preliminary assessment result of prothioconazole for endocrine disruption also evidences that it still has excellent market prospects.
However, it should also be noted that even though prothioconazole is already in the off-patent period, Bayer still has global technical barriers to trade and an absolute market power through its deep involvement in renewal assessment in the EU and the US.
If you are interested in the renewal assessments of prothioconazole and other pesticide varieties in the EU and the US, please contact REACH24H Consulting Group via customer@reach24h.com for consultancy. For more services, please visit https://www.reach24h.com/en/agrochemicals.