Abbott Fined Nine Million Yuan for the Detection of Vanillin in Stage 1 Infant Formula
Takehome:
- The tiny amount of vanillin detected in the involved batch of Abbott’s stage 1 infant formula is mainly the residues in pipeline after the production of another batch of products.
- In 2019, 13 domestic infant formula companies in China initiated an “Anti-flavoring Action” and promised to rule out the use of flavorings in infant formula products.
On May 6, 2021, Shanghai State Administration of Market Regulation (SAMR) issued the decision to fine Abbott Laboratories Trading (Shanghai) Co,.Ltd. over 9.09 million yuan (around USD 1.4 million dollars) for the detection of vanillin, a food flavoring, in its stage 1 infant formula. Besides, a total of 3.43 million yuan of illegal income would be confiscated as well. The reason behind this penalty is that according to the regulations1 in China, vanillin is a forbidden substance in stage 1 infant formula (for infants from 0-6 months) and can only be used in stage 2 and 3 infant formula products (for babies from 6-12 months and 12-36 months, respectively) at a maximum of 5mg/100mL.
On May 14, 2021, Shanghai SAMR unveiled the notice2 of this administrative penalty decision. It disclosed that the tiny amount of vanillin, 189.0 µg/kg, detected in the involved batch (Batch No.: 18042NT of Abbott Similac Plus Infant Formula Stage 1) was mainly the residues in pipeline after the production of another batch of products. Although the manufacturer did not add vanillin into products intentionally, these products were still contaminated during the production, which violates the Food Safety Law3 in China.

Image: National Enterprise Credit Information Publicity System
Suggestions for Stakeholders
Rock Luo, a senior technician of Reach24h Consulting Group expounded that manufacturers should follow the manufacturing practice strictly during the production. When several products share the same production line, recipes with simple ingredients or without odor should be manufactured first or manufacturers shall ensure the ingredients used in this batch of products can be further used in the next batch. To cases like Abbott (vanillin can be used in the previous batch of products but cannot be used in the next batch), the production pipeline must be cleaned thoroughly.
Apart from the quality control, consumers demands is another issue worth noting. Despite the legal use and widely application of food additives (including flavorings) in daily products, many consumers still believe the massive use of food additives is one of the major concerns4 in terms of food safety. Under the comments of relevant reports about this case, ChemLinked notes that after knowing vanillin is a kind of food flavoring, many moms are eager to find the substitute without vanillin for the stage 2 and 3 infant formula products they used to purchase.
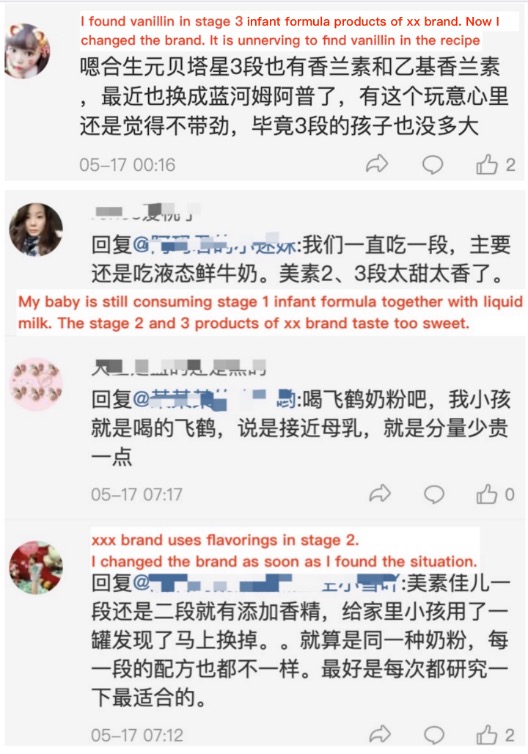
Source: Sina Weibo
Is it a must to add vanillin in infant formula products? Jiang Jingxiong, a nutrition expert of China Healthy Birth Science Association pointed out5 that a healthier infant formula recipe should not include unnecessary ingredients like flavorings, sucrose, maltodextrin, etc. Take vanillin as an example, vanillin does not provide nutritional value. The addition of vanillin in infant formula is mainly to give milk powder a good taste. However, infants will be addicted to this taste after a long-term consumption, and may become picky eaters after growing up. Out of similar reasons, in 2019, 13 domestic infant formula companies6 in China, including Junlebao, Firmus, etc., initiated an “Anti-flavoring Action” and promised to rule out the use of flavorings in infant formula products. Industrial experts also indicated that at present, most domestic brands have banned the use of flavorings like vanillin, but it can still be seen in many foreign brands. Hence, ChemLinked’s suggestion is stakeholders should evaluate such demands and consider to advance the recipe accordingly, especially when the market is facing a fierce competition. After all, the composition of infant formula recipe will highly influence consumers preference.
Visit ChemLinked for more updates and analysis of China’s infant formula industry.
Reference Link
- The requirements of using vanillin in infant formula, ChemLinked Food Additive Database
- Notice of the administrative penalty decision
- China’s Food Safety Law, ChemLinked Regulatory Database
- Chinese consumers attitudes towards food additives
- Experts’ opinions on the use of vanillin
- 13 domestic infant formula companies initiated an anti-flavoring action


