China New Chemical Substance Registration(MEE Order No. 12)
Overview
The Measures for the Environmental Management Registration of New Chemical Substances (Order No. 12 of the Ministry of Ecology and Environment of the P. R. China, MEE Order No. 12) was released in April 2020 and came into effect from January 1, 2021. It has replaced the Measures on the Environmental Management of New Chemical Substance (MEP Order No.7) and become a brand-new management standard for new chemical substances in China.
MEE Order No. 12 shall apply if companies plan to manufacture or import chemicals not listed in the Inventory of Existing Chemical Substances in China (IECSC). Registration dossiers should be submitted to the Solid Waste and Chemicals Management Center of MEE (SCC-MEE) and apply for approval. A certificate should be granted before any new chemical substance enters the China market, otherwise, relevant companies will be subject to severe penalties.
IECSC was last updated on November 25, 2024.
China New Chemical Substance Registration (China REACH) includes regular registration, simplified registration, record registration, and new usage management registration.
Scope of Applicable Substances
MEE Order No. 12 requires the registration of “new” chemical substances in China. New chemical substances refer to chemical substances that are not listed in the Inventory of Existing Chemical Substances of China ( IECSC), including:
- New chemical substances that are pharmaceuticals (including active pharmaceutical ingredients), pesticides (including pesticide technical), veterinary drugs(including active pharmaceutical ingredients), cosmetics, food, food additives, feed, feed additives, fertilizers and other products that are to be used in other industrial applications, and those used as raw materials or intermediates for the production of the aforementioned products;
- New chemical substances contained in surfactants, plasticizers, preservatives, dispersants, flame retardants and other products with specific functions, or their preparations;
- Polymers;
- New chemical substances contained in articles that are intended to be released in general use;
- Intermediates that are not non-isolated intermediates;
- etc.
Chemical substances which have been listed in the IECSC and subject to new usage environmental management, and are used for industrial applications other than the permitted uses, including:
- Highly hazardous chemical substances;
- Chemical substances with persistence and bioaccumulation (PB), or persistence and toxicity (PT), or bioaccumulation and toxicity (BT) properties.
Exemptions
- Pharmaceuticals (including active pharmaceutical ingredients), pesticides (including pesticide technical), veterinary drugs(including active pharmaceutical ingredients), cosmetics, food, food additives, feed, feed additives, fertilizers, and other products;
- Radioactive Substance;
- Naturally occurring substances;
- Non-commercial or non-intentionally produced substances: impurities, by-products, wastes, etc;
- Other special categories: alloy, non-isolated intermediates, articles, the mixture of existing chemicals that are artificially blended and without producing new chemicals, an anhydrous chemical and its hydrate, one of which has been listed in the IECSC, etc.
Registration Bodies
- Manufacturers/direct importers of new chemical substances in China;
- Overseas companies should designate a representative agent when applying for the registration;
- Manufacturers, importers or processors, or users can be the applicant where the exempted products specified in Article 2 of the Measures are new chemical substances and are intended to be changed to other industrial applications;
- Manufacturers, importers, processors and users can be the applicant where the chemical substances subject to new usage environmental management are intended to be used for industrial applications other than permitted uses, or for highly hazardous chemical substances that have not obtained the regular registration certificate for the intended use.
Note: Companies from Hong Kong, Macau and Taiwan shall appoint a Chinese Mainland representative agent to complete the registration.
Registration Types
| Record Registration | 1) New chemical substances manufactured or imported with an annual volume of less than 1 ton;
2)Polymers containing less than 2% monomers or reactants which are new chemical substances, or polymers of low concern (no volume limit), including: a) Polymer itself is not listed in the IECSC, but all the new monomers or reactants of the polymer are ≤2% (w/w); b) Polymer itself is not listed in the IECSC, but all the monomers or reactants of the polymer are listed in the IECSC; c) The polymer’s number-average molecular weight is between 1,000 and 10,000 daltons. The content of oligomers in the polymer with molecular weight <500 daltons is less than 10% (w/w) and the content of oligomers with molecular weight <1000 daltons is less than 25% (w/w). At the same time, the polymers must not contain functional groups of high concern or high reactivity; d) The polymer’s number-average molecular weight is ≥10,000 dalton. The content of oligomers in the polymer with molecular weight <500 daltons is less than 2% (w/w) and the content of oligomers with molecular weight of <1,000 daltons are less than 5% (w/w); e) Polyesters. New chemical substances not conforming to the record registration conditions for polymers or meeting the exclusion requirements for polymers shall be subject to regular or simplified registration. |
| Simplified Registration | 1 t/a ≤ Annual Manufacturing / Importation Volume < 10 t/a |
| Regular Registration | Annual Manufacture / Importation Volume ≥ 10 t/a; |
Registration Period
| Registration Types | Registration Period |
| Record Registration | 1~2 Weeks |
| Simplified Registration | 8~12 Months |
| Regular Registration | 14~24 Months |
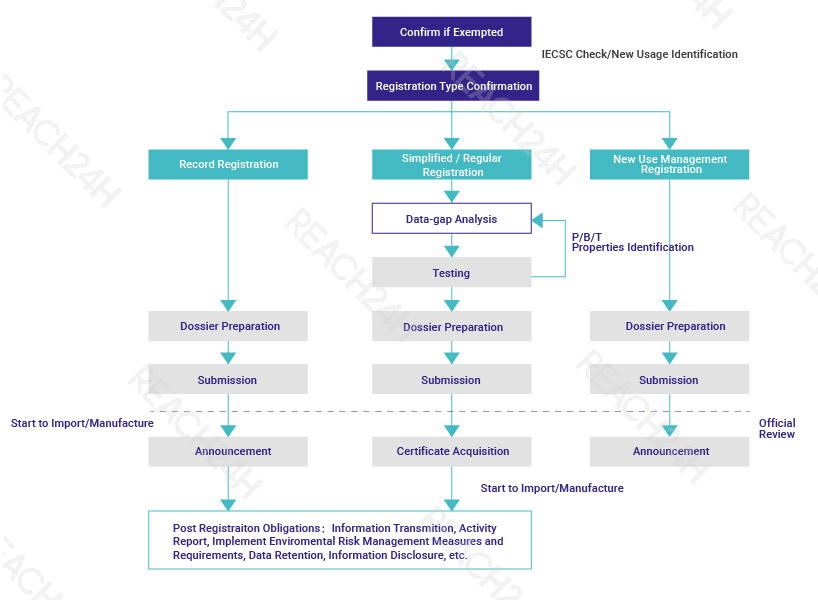
Registration Procedure
Post-registration Obligations
According to the registration type of new chemical substances, the corresponding post-registration obligations are not exactly the same. The holder of the new chemical substance registration certificate and the processors or users generally need to fulfill the following obligations:
| Type | Post-registration Obligation |
| General Requirement | The registration certificate holders, record registration applicants and processors or users shall carry out activities in accordance with the matters specified in the registration certificate or the record registrations. The registration certificate is not transferable.
The researchers, manufacturers, importers, processors and users of new chemical substances shall implement relevant environmental risk control measures and environmental management requirements. |
| Information Transmission | The manufacturers, importers, processors and users of new chemical substances shall transmit the following information to downstream users:
1. The New Chemical Certificate no. or the Record Receipt no.; 2. Registered Uses of new chemical substances; 3. Environmental and health hazard properties, and environmental risk control measures for the new chemical substances; 4. Environmental management requirements specified on the registration certificate. Manufacturers, importers, and processors and users may transmit the above information to downstream users, and request them to continue the transmission level by level to the final processors and users. At the same time, processors and users can also request suppliers to provide the above relevant information. |
| First Activity Report | The holder of the regular and simplified registration certificate or their designated agents shall submit the first activity report of the new chemical substance within 60 days from the date of the first production activity or the first import and transport to the processors and users. |
| Annual Report | If submitting of an annual report is specified as an environmental management requirement on the regular registration certificate, the registration certificate holder or its designated agent shall submit an annual report of new chemical substances before April 30 each year. |
| Information Transparency | The manufacturers, processors and users of the regular registration new chemical substances should disclose the implementation of environmental risk control measures and environmental management requirements through their official websites or other methods convenient for the public to access, and timely update. |
| New Hazard Information And Environmental Risk Tracking | Researchers, manufacturers, importers, processors and users of new chemical substances shall timely report to the MEE when they discover new environmental or health hazard properties or environmental risks of new chemical substances. If it may lead to an increase in environmental risks, prompt measures shall be taken to eliminate or to reduce environmental risks. |
|
Data Retention |
Researchers, manufacturers, importers, processors and users of new chemical substances shall establish a record system for activities of new chemical substances, which shall truthfully record the activity time, quantity, actual uses, and implementation of risk control measures and requirements. Materials of regular and simplified registration, and activity records shall be kept for at least 10 years, and record materials and activity records shall be kept for at least 3 years. |
Competent Department
The competent department of ecology and environment under the State Council shall notify the competent department of ecology and environment at the provincial level the information on registration, environmental risk control measures, environmental management requirements, first-time activities, and annual report, etc. of the new chemical substances. The competent department of ecology and environment at the provincial level shall also notify the above information to the competent department of ecology and environment at the district level.
The competent department of ecology and environment at or above the level of a districted city shall conduct supervision and spot check on the activities of new chemical substances, focusing on whether the manufacturers, importers, processors, and users of the new chemical substances handle the environmental management registration of new chemical substances as requires, the authenticity of the registration items, the items specified in the registration certificate, and implementation circumstances of other relevant provisions in the Measures.
Researchers, producers, importers, and processors and users of new chemical substances shall truthfully provide relevant information and accept the supervision and random inspection of the MEE.
Our Services
- Comprehensive Regulatory Compliance Consulting
- Representative Agent Service
- IECSC Comprehensive Search
- Inventory Listing Application
- New Usage Management Identification
- New Chemical Substance Registration (Record Registration/Simplified Registration/Regular Registration/New Usage Management Registration)
- Overall Registration Scheme
- Data Assessment/Data Gap Analysis/Exemption Analysis/PBT Properties Identification
- Non-testing Assessment Report (Toxicokinetics Assessment Report, QSAR, Read-across)
- Test Monitoring
- Risk Assessment Report / Social Economic Effectiveness Analysis Report Preparation
- Communication with Competent Authorities/Experts
- Translation for Registration Documents
- Post-registration Obligations (Annual Report, Certificate Renewal, etc.)
- Customized Training
Why REACH24H?
- Rich Experience: Above 10-year practical experience in the field of China’s new chemical management regulation.
- Outstanding Performance: As of December 2021, REACH24H has successfully submitted more than 3,000 applications under MEP Order No. 7, including dozens of high tonnage difficult substances, and the pass rate is 100%.
- Strong technical strength: We have a team of above 30 senior technical personnel with academic and professional backgrounds in toxicology, analytical chemistry, chemical engineering, biology, pharmacology, environment, etc.
- Internationalized service: Our customer service team consists of personnel capable in English, Chinese, Japanese, Korean and German to efficiently serve multi-national customers.
- Rich accumulation: Accumulated rich experience in dealing with global chemical regulatory compliance and technical consulting.
- Abundant Testing Facility Resources: Well-established collaboration with a wide range of certified testing facilities.
- Close relationship and good communication channel with competent authorities and experts.