REACH24H Successfully Completes Product Authorizations In The EU For 13 Companies In One Month
In December 2018, REACH24H Consulting Group China successfully completed the union authorization of EU-BPR for two Asian companies, and successfully provided 13 European companies with support for union authorization/ same biocidal products authorization. This means that the companies represented by REACH24H have successfully crossed the technical trade barriers and taken an important step towards expanding in the EU market.
The active substance involved in this product authorization is calcium hypochlorite (CAS: 7778-54-3), which covers the following uses:
- PT2: Disinfectants and algaecides not intended for direct application to humans or animals;
- PT3: Veterinary hygiene;
- PT4: Food and feed area;
- PT5: Drinking water.
On December 14, 2016, the Biocidal Products Committee (BPC) issued a resolution approving calcium hypochlorite for PT 2, 3, 4, 5. The EU Chemicals Agency (ECHA) subsequently issued a product authorization deadline for the product containing the active substance calcium hypochlorite on January 1, 2019 (see Figure 1).
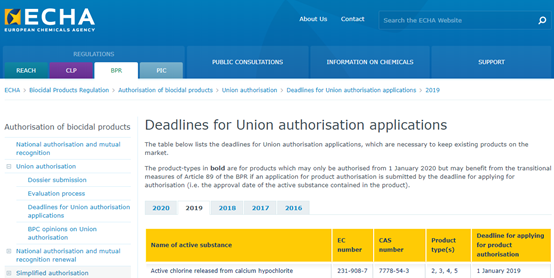
FIG. 1 The deadline for EU union authorization of calcium hypochlorite products
EU BPR, which was formally implemented on September 1, 2013, not only has a long compliance cycle, heavy workload and high cost, but also strict and complex compliance requirements. Despite of the tight deadline for calcium hypochlorite, with the years of technical precipitation and project experience, REACH24H has maintained regular and efficient communications with European officials, specifically senior experts from technical teams, to give full support, and tailored solutions for customers to successfully solve the urgent corporate needs. As a happy result, in just a month REACH24H EU BPR team successfully assisted 13 European companies to complete the union/ national authorization compliance work for one biocidal product, and the team was proud to achieve this in a reduced time and at a lower cost.
The completion of BPR registration can not only well protect the interests of the claimant, but also greatly improve the market competitiveness of enterprises, allowing them to sieze the opportunity to explore the EU market, and achieve greater economic benefits. So far, EU BPR regulatory team in REACH24H has helped companies complete more than 100 EU applications, including nearly one hundred product authorization applications and over a dozen active substance supplier list applications and active substance approvals. In addition, the team has also supported a number of technical equivalence assessments. Last but not least, they have helped some small enterprises reduce their EU authority administration fee by applying for the prescribed SME discount (small and medium-sized enterprise) for evaluation applications. All in all, December was a truly challenging and rewarding month in terms of EU BPR.
About REACH24H Agrochem Department: Providing a bridge and link between European Union officials and Asian companies, REACH24H actively speak out on behalf of non-EU companies, enabling them to explore the trade of biocides in the EU and around the world. If you have further questions, please feel free to contact us!
REACH24H Agrochem Department: +86 571-87006630
Email: customer@reach24h.com




